![]()
You are here
University Hospital Galway Achieves Global First with Revolutionary Device for Treating Complex Venous Obstructions
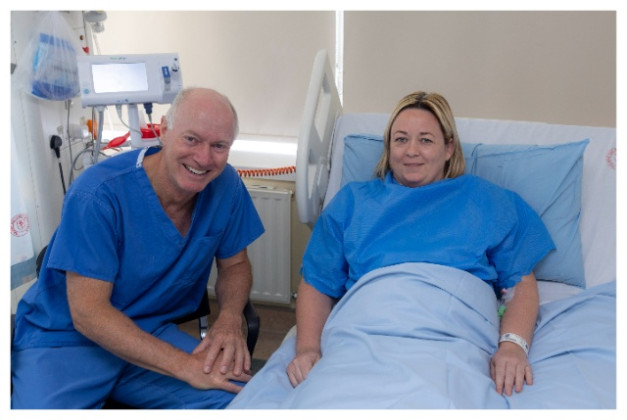
University Hospital Galway has achieved a global medical breakthrough by becoming the first hospital in the world to successfully trial a revolutionary new catheter device, designed to treat complex venous obstructions, particularly blocked or damaged leg veins that have previously been stented and fail to return blood efficiently to the heart.
The Recana Thrombectomy Catheter System, developed by InterVene, Inc., is the first fully integrated mechanical thrombectomy device specifically engineered to treat in-stent restenosis, a condition in which veins narrow again after stenting. The minimally invasive device is inserted through a small incision in the leg, allowing clinicians to access and clear previously obstructed veins. This restores healthy blood flow and offers new hope to patients who have exhausted all conventional treatment options.
The first patient to receive this transformative treatment was Kelly Coughlin from Ballyshannon, County Donegal. Her successful treatment marks a major milestone in the management of chronic venous disease and highlights the strength of the collaboration between University Hospital Galway, University of Galway’s Institute for Clinical Trials, and InterVene, Inc.
“This marks a significant advancement in the treatment of chronic venous disease,” said Professor Gerry O’Sullivan, Consultant Interventional Radiologist at University Hospital Galway, who led the procedure.
“The long-term impact of venous obstructions, especially in previously stented vessels, can be life-altering. I had attempted to recanalize this patient’s veins four times without success. The Recana system achieved this in a single session. That speaks volumes about its potential.”
“It offers a powerful, minimally invasive solution for patients who have exhausted conventional treatment options,” added Professor O’Sullivan.
Kelly Coughlin, the first recipient of the treatment, shared her experience: “This procedure has been massively life changing for me. When I was symptomatic, even simple tasks like walking were a challenge.
"I’m incredibly grateful to Professor O’Sullivan and his team at University Hospital Galway. I’ve been under his care for many years, and that long-standing trust gave me the confidence and reassurance I needed to proceed with this treatment.”
Chris Kane, Hospital Manager, praised the achievement, stating: “This is a significant achievement for University Hospital Galway and reflects our unwavering commitment to clinical excellence and innovation. Being the first in the world to trial this ground-breaking device showcases both the expertise of our medical teams and the strength of our collaborative research partnerships.”
Professor Fidelma Dunne, Director of the Institute for Clinical Trials at University of Galway, praised the collaborative success: “It is incredibly rewarding to see cutting-edge medical device innovation introduced in Ireland through the Hypercare initiative at University of Galway’s Institute for Clinical Trials.
“Hypercare was developed to streamline and accelerate the start-up of clinical trials by working closely with our regulatory, ethical, and healthcare delivery partners. This milestone is a clear reflection of that mission in action.”
This ground-breaking procedure highlights University Hospital Galway’s position at the forefront of global medical innovation, clinical research, and collaborative healthcare, establishing a new standard in the treatment of complex venous disease.
The article above is specific to the following Saolta hospitals::
University Hospital Galway (UHG)
Keywords:
